study register
A study register provides an up-to-date and user-oriented overview of studies. The freely available information in the national and international study registers supports transparency in medical research.
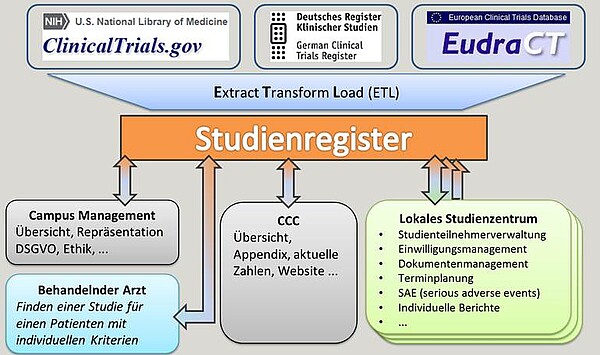
A prerequisite for a UKSH-wide clear presentation of all studies and the associated recruitment is the registration of all studies in one portal. The ITCR-L, together with local study centres (e.g. surgery, haematology) and the ICB-L, has created a study register for all study activities within the UKSH (Campus Lübeck) and provided the most important information for interested doctors and staff. The study register is updated on a monthly basis via the export and import options. By assigning the studies to the study centres, it is possible to trace the contents back to the websites of the individual clinics or study centres. The visibility of research at the UKSH and the UzL is increased and patients and other interested parties can view current studies.
study management
Cooperation with study centres has shown that the management of the general process flows of a study is so complex that local "IT auxiliary solutions" reach their limits. It was possible to develop a generic procedure using CentraXX software, with which different processes of different centers can be supported very effectively. In addition, reports with upcoming appointments for visits in the coming week or month can be sent by e-mail. In CentraXX all information is depictable thereby, which is needed for the management of studies.
Study documentation using a CTMS (Clinical Trial Management System)
The ITCR-L offers its customers a complete clinical trial management system that supports the study manager and study team in the planning, preparation, conduct and evaluation of clinical trials. The main task is structured data collection and storage, which form the basis of study data storage, reporting and analysis. In addition, plausibility checks, data backup, access controls, randomization and other functionalities are provided.